How To Correct For Fluorescence Background In Raman Data In Origin
Photobleaching profile of Raman peaks and fluorescence groundwork
Laser-induced fluorescence is the most common source of interfering baseline betoken encountered in Raman measurements. It shows itself as a slowly changing groundwork in a spectrum. Ane of the challenges in the successful utilise of Raman spectroscopy is to extract Raman signatures from this, orders of magnitude stronger, broadband fluorescence emission. Irradiating the sample with intense laser light, ie, photobleaching, is one effective technique to reduce the level of fluorescence emission, thus increasing the indicate to noise (S/N) ratio of a spectrum.

The fluorescence interference in Raman spectroscopy may issue from the compound analysed or from fluorescent impurities in the sample. It is an assimilation process that causes molecules to exist excited to a higher electronic state, which requires high-energy photons. Fluorescence light is then emitted while molecules relax back to the lower free energy level. The phenomenon depends strongly on the excitation wavelength and appears only at a stock-still frequency, while the Raman shifts are independent of the laser's wavelength.
Raman scattering and fluorescence emission may compete with each other when the excitation laser energy is close to the electronic transition energy of the fabric. Higher energy green or red excitation sources, such equally 514nm or 633nm visible laser, produce stronger fluorescence groundwork. As the light amplification by stimulated emission of radiation line moves to the most-infrared (NIR) region, as with 785nm or 1,064nm laser, the fluorescence outcome subsides or completely disappears since the energy of these wavelengths may not be sufficient to excite the molecule to college electronic state or may not be enough to destroy fluorescing molecules in the cloth. This effect is illustrated in Figure 1.
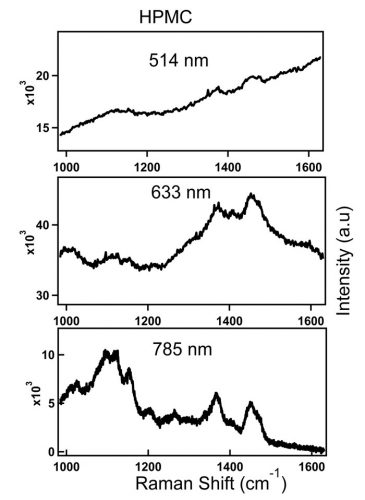
Figure 1: Spectra of HPMC obtained with 514nm, 633nm and 785nm laser lines. Top spectrum (514nm): 5s, 77mW at the sample. Middle (633nm): 30s, 33mW at the sample. Bottom (785nm): 60s, 68mW at the sample.
Effigy one shows spectra of an inactive pharmaceutical ingredient, hydroxypropyl methylcellulose (HPMC), a common pharmaceutical excipient, which is measured at three different laser lines; 514nm, 633nm and 785nm. The figure illustrates the loss of Raman peaks in the presence of increasing levels of fluorescence with loftier energy excitation sources. In contrast to the 514nm laser excitation line that produced significant fluorescence baseline, a better S/North ratio HPMC spectrum is acquired with 785nm excitation wavelength. Advances in compressive detection strategy accept recently been fabricated to facilitate Raman nomenclature and quantitation in the presence of fluorescence background.i This is reported to be a better alternative to conventional subtraction strategies2-4 by virtue of its compatibility with automated high-speed chemical analysis in the presence of fluorescence background.1
It is well known that Raman scattering is an inefficient phenomenon. Typically, one Raman photon is generated for every 106 to 109 laser photons incident upon the sample. Equally a outcome, very low amounts of fluorescent species in the sample may be enough to mask the low Raman scattered photons or make it difficult to interpret the spectrum. If the fluorescence baseline is high, the shot racket generated by this betoken may exist of a similar order, or even greater than the Raman signal alone and will completely mask the betoken from the Raman photons. Consequently, proposed mathematical techniques2-iv to eliminate the fluorescence background will but make larger peaks more than visible against the groundwork while smaller peaks may still remain undiscernible against the noise.
In many cases, Raman signal can still exist acquired from samples producing autofluorescence. In the fluorescence process, the sample is excited to the college electronic state by the absorption of a photon and subsequently relaxes dorsum to the basis electronic state past emitting a fluorescence photon. When compared to the rate of fluorescence emission, which typically occurs in 10-5 to 10-10s, the rate of photon absorption is very rapid at about 10-14 to 10-15s. Thus, the fluorescence effect can exist reduced past photobleaching the sample. Alternatively, if the fluorescence is due to an impurity in the material, long exposure to sustained laser light may destroy fluorescent impurities, hence decreasing the fluorescence background. More specifically, irradiating the sample with an intense light amplification by stimulated emission of radiation axle for a catamenia of time before data acquisition could remove or partially quench the fluorescence, leading to a higher quality Raman spectrum. The irradiation fourth dimension menstruum tin range from seconds to hours, depending on the fluorescent material in the particular sample.
Experiment and results
In this written report, pure microcrystalline cellulose (MCC) was photobleached for about 1hr and sequent Raman spectra were recorded at various time periods without moving the sample. These measurements were carried out with an in-firm constructed Raman musical instrument. The sample was irradiated with a unmarried manner diode light amplification by stimulated emission of radiation of 785nm wavelength, which delivered 80mW of ability at the sample. A 20x NIR objective lens (NA 0.40, Olympus, LMPL 20X IR) was used to focus the light amplification by stimulated emission of radiation onto the sample and besides to collect scattered Raman photons. The scattered light was dispersed with a 300 lines/mm grating. Acquisition time for each spectrum was 60s. A total of twoscore spectra were collected in a time span of 61min. Information assay was carried out using Igor Pro 6.37 (WaveMetrics).
The spectrum of MCC has a high fluorescent groundwork, which bleaches downwards in time. Figure 2 shows the representative spectral fourth dimension series obtained at half dozen unlike time points to demonstrate the overall effect of photobleaching on the spectra. The Raman peaks are located on acme of the fluorescence groundwork in the spectra. Earlier any adding, a nighttime background is subtracted from each spectrum. In order to calculate the areas of Raman peaks and fluorescence background, Raman peaks betwixt 765cm-1 and 1,580cm-ane spectral range are fitted to 3rd-caste polynomial equally shown in Figure 3A. Then, these Raman spectral features are effectively separated from the fluorescence background and the expanse shown above red polynomial curve in Figure 3A is calculated equally a Raman elevation area.
Too, the fluorescence counts over the 500cm-one to 3,410cm-1 range without including the Raman peaks are calculated for each spectrum collected, so that the level of fluorescence emitted tin be evaluated equally a part of photobleaching time. Figure 3B gives the photo-bleaching profile, demonstrating how the intensities of fluorescence background and Raman peak areas change with light amplification by stimulated emission of radiation exposure at unlike periods of irradiation fourth dimension. The left axis in Figure 3B corresponds to the curve of fluorescence area calculated at each irradiation time. The curve is fitted to a double exponential function (red line), indicating that the fluorescence level decreased at the beginning in more than rapid mode than ensuing time periods. The correct axis of the graph represents the Raman top areas. While fluorescence area shows an exponential decrease, no systematic (accompanying) change was observed in Raman peak area equally the photobleaching progressed. At that place were no changes in peak positions, elevation broadening or any changes in general shape. Thus, it is reasonable to infer that Raman photons are not afflicted by continuous laser exposure and remain unchanged throughout.
Concluding remarks
Resonant interaction of an heady laser with electronic sample states, which produces orders of magnitude college intensity fluorescence groundwork than that of Raman signatures, is the well-known nemesis of Raman spectroscopy. If the sample exhibits a fluorescence effect, in most cases, the effect may exist so intense that the Raman signatures cannot be detected, thus precluding a reliable assay. Subjecting a specimen to prolonged photobleaching by continuously irradiating it with laser low-cal is demonstrated to reduce the fluorescence background exponentially, without affecting the Raman peak area or the Raman peak heights calculated from the absolute peak height in a higher place the local groundwork.
Acknowledgment
The present work was financially supported by NSF-Engineering science Inquiry Heart for Structured Organic Particulate Systems (ERC-SOPS, EEC-0540855). The authors also acknowledge Professor Rodolfo J Romañach at the Academy of Puerto Rico – Mayagüez Campus, for his conscientious review of the manuscript.
References
- Rehrauer OG, Mankani BR, Buzzard GT, Lucier BJ, Ben-Amotz D. Fluorescence modeling for optimized-binary compressive detection Raman spectroscopy. Optics Express. 2015;23(18):23935-23951.
- Lieber CA, Mahadevan-Jansen A. Automated Method for Subtraction of Fluorescence from Biological Raman Spectra. Applied Spectroscopy. 2003;57:1363-1367.
- Beier BD, Berger AJ. Method for automated background subtraction from Raman spectra containing known contaminants. Analyst. 2009;134(6):1198-1202.
- Zhao J, Lui H, McLean DI, Zeng H. Automated autofluorescence background subtraction algorithm for biomedical Raman spectroscopy. Practical Spectroscopy. 2007;61(11):1225-1232.
Biography
 DERYA CEBECI-MALTAŞ is currently with PortMera (in Technopark Istanbul) every bit a Research Chemist, applying vibrational spectroscopy and chemometrics to develop realtime measurement systems for continuous pharmaceutical manufacturing. She has a PhD in Analytical Chemistry from Purdue University and holds an MBA from Ball State University. Prior to joining PortMera, she worked at the FDA as a postdoctoral fellow, generating spectroscopy methods for at-field counterfeit screening applications.
DERYA CEBECI-MALTAŞ is currently with PortMera (in Technopark Istanbul) every bit a Research Chemist, applying vibrational spectroscopy and chemometrics to develop realtime measurement systems for continuous pharmaceutical manufacturing. She has a PhD in Analytical Chemistry from Purdue University and holds an MBA from Ball State University. Prior to joining PortMera, she worked at the FDA as a postdoctoral fellow, generating spectroscopy methods for at-field counterfeit screening applications.
 Dr. ANIK ALAM is currently pursuing his PhD degree in Pharmaceutics from Duquesne Academy. He has been working on the development of multivariate calibration in spectral space for quantitative analysis of pharmaceutical tablets. His inquiry activities also include the implementation of procedure analytical engineering and quality by design principles in pharmaceutical manufacturing fix-upwardly to monitor and control unit operation processes.
Dr. ANIK ALAM is currently pursuing his PhD degree in Pharmaceutics from Duquesne Academy. He has been working on the development of multivariate calibration in spectral space for quantitative analysis of pharmaceutical tablets. His inquiry activities also include the implementation of procedure analytical engineering and quality by design principles in pharmaceutical manufacturing fix-upwardly to monitor and control unit operation processes.
 PING WANG received his PhD from Wuhan Plant of Physics and Mathematics, Chinese University of Science. He did postdoctoral research at Purdue University's Departments of Physics (2008-2010), Chemistry (2011-2012), and Biomedical Applied science (2012-2015). He is currently Professor at Wuhan National Laboratory for Optoelectronics. His research focuses on labelfree chemical imaging of cultured cells, unprocessed tissues, alive organism and human diseased tissues by stimulated Raman handful microscopy.
PING WANG received his PhD from Wuhan Plant of Physics and Mathematics, Chinese University of Science. He did postdoctoral research at Purdue University's Departments of Physics (2008-2010), Chemistry (2011-2012), and Biomedical Applied science (2012-2015). He is currently Professor at Wuhan National Laboratory for Optoelectronics. His research focuses on labelfree chemical imaging of cultured cells, unprocessed tissues, alive organism and human diseased tissues by stimulated Raman handful microscopy.
 DOR BEN-AMOTZ obtained his PhD from UC Berkeley, followed past a postdoctoral fellowship with Dudley Herschbach at the Exxon Corporate Enquiry Lab in Annandale, NJ. He has been a faculty member in the Department of Chemistry at Purdue University since 1989. His recent experimental and theoretical interests include hydration-shell spectroscopy, liquid theory, hyperspectral compressive imaging, and new means of didactics physical chemical science.
DOR BEN-AMOTZ obtained his PhD from UC Berkeley, followed past a postdoctoral fellowship with Dudley Herschbach at the Exxon Corporate Enquiry Lab in Annandale, NJ. He has been a faculty member in the Department of Chemistry at Purdue University since 1989. His recent experimental and theoretical interests include hydration-shell spectroscopy, liquid theory, hyperspectral compressive imaging, and new means of didactics physical chemical science.
 RODOLFO PINAL is Associate Professor of Industrial and Physical Pharmacy at Purdue University. Prior to his bookish appointment, he worked at Hoffmann-La Roche (Nutley, NJ) for xiii years, where he was Head of the Solid-State Characterisation Laboratory. His research interests include the study of raw material functionality and manufacture of personalised medications.
RODOLFO PINAL is Associate Professor of Industrial and Physical Pharmacy at Purdue University. Prior to his bookish appointment, he worked at Hoffmann-La Roche (Nutley, NJ) for xiii years, where he was Head of the Solid-State Characterisation Laboratory. His research interests include the study of raw material functionality and manufacture of personalised medications.
How To Correct For Fluorescence Background In Raman Data In Origin,
Source: https://www.europeanpharmaceuticalreview.com/article/70503/raman-peaks-fluorescence-background/
Posted by: westbrookwhanderharty.blogspot.com


0 Response to "How To Correct For Fluorescence Background In Raman Data In Origin"
Post a Comment